Cleaning Validation Analysis Made Easy

45 minutes
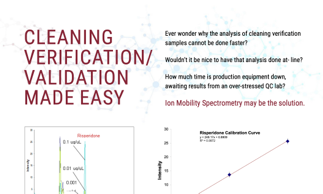
Cleaning validation/verification is an FDA requirement. Inadequate or incomplete cleaning validation is one of the leading causes of internal investigations and regulatory citings. Current technologies keep production equipment off line as they are either too slow, not specific, inconvenient, or not sensitive enough.
This webinar introduces ion mobility spectroscopy (IMS) as a GMP-compliant alternative to legacy methods for at-line cleaning verification, such as TOC swab analysis and HPLC. There has been a lot of talk about the use of hand-held devices for at-line analyses, however, these are not suitable for a GMP environment. Legacy techniques are associated with long instrument downtime, and lack of efficiency. IMS addresses all of these shortcomings and more.

Before joining Excellims, Dave held several sales and sales management positions including Pion Inc., Gerstel, Pioneer Analytical, ThalesNano Inc., and Distek Inc. Dave has attained 16 years of success: growing territories, building sales teams, developing and launching products and serving as a business owner with executive management responsibility. While sales started out as a job, it has turned into Dave’s career and passion.
Dave has a BS in Chemistry from the College of the Holy Cross.
Outside of work, Dave spends most of his free time with his family, engaging in a range of outdoor activities such as fishing, golfing and skiing.

Ching has been working on all aspects of ion mobility spectrometry and mass spectrometry technology for over 25 years, from initial instrument design/product development to exploring new applications and business development. Before founding Excellims, he served as the Research Leader for GE Security's chem/bio and explosives detection business, he was responsible for many government funded and GE internal programs to support the business growth. He also served as Application Manager, managing a team of scientists and engineers, directly supporting business development and sales activities. Previously, Ching served as mass spectrometry Software Manager at Bruker Daltonics managing software development projects for Bruker’s time of flight mass spectrometer product lines.
He has both Ph.D. and MS degrees in Chemistry from Washington State University, an MS in Computer Science also from Washington State University, and an MS in Chemical Engineering from Yokohama National University.
Ching has authored more than 50 peer reviewed papers and patents/patent applications in ion mobility spectrometry and mass spectrometry field.